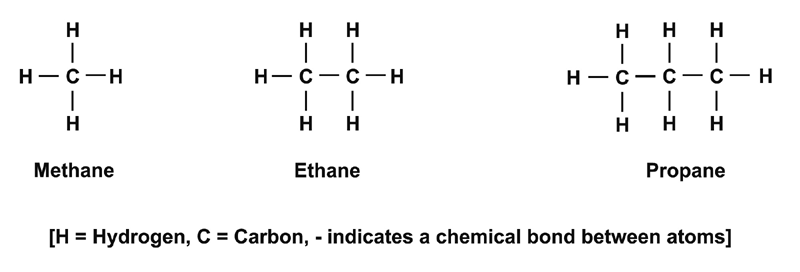
1. Intramolecular bonds = within a molecule (*think intramural)
- Ionic + Covalent
2. Intermolecular bonds = between molecules (*think international)
- The stronger the intermolecular bonds the higher the BP or MP
- 2 Types: Vander Waals bonds & Hydrogen bonds
Vander Waals Bonds:
- Based on electron distribution
- 2 Categories:
- Dipole - Dipole bonds
- if a molec. is Polar, the + end of one molec will be attracted to the - end of another molec.
2. London Dispersion Forces (LDF)
- present in all molecs
- creates the weakest bonds
- if a substance is non-polar Dipole - Dipole forces don't exist
- electrons are free to move around & will randomly be grouped on one side of the molec.
- Creates a temporary dipole and can cause a weak bond to form
- the more e- in the molec. the stronger the LDF will be
- NH3 (10e) VS. C2H8 (18e)
polar non-polar
- NH3 has the stronger bond because of it's Dipole-Dipole bond. C2H8 is non-polar thus it's bond is LDF making it's bond weaker than NH3.
Hydrogen Bonding:
- if hydrogen bonded to certain elements (F, O, & N) the bond is highly polar
- this forms a very strong intermolecular bond.
- H2O (10e) VS. CH4 (10e)
Polar Polar
- They are both Polar and both have the same amount of e- but H2O wins for the highest boiling point due to its Hydrogen bond.
A very helpful Video... :)