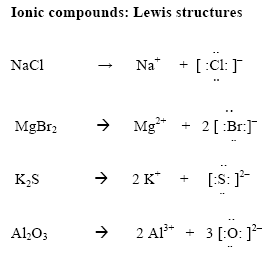- The mass (grams) of 1 mole of a substance is called the molar mass
- It can be determined from the atomic mass on the periodic table
- Measured in g/mol
So what exactly is Molar Mass?
Molar mass is the
atomic weight of an element expressed in grams is the mass of 1 mole of element.
How do you determine Molar Mass in a Compound?
To determine the molar mass of a compound you must
ADD all the mass of the atoms together
Here's an explanation that will help you understand what Molar mass is:
Follow these steps to find molar mass!
What is the molar mass of H20? (water)
- Identify how many atoms of each of the elements are in the chemical formula. In the example there are 2 atoms of Hydrogen and there is 1 atom of Oxygen.
- Look at the periodic table and search for the elements given & then look at each of their atomic masses. The elements in H20 are Hydrogen and Oxygen. The atomic mass for Hydrogen is 1.0 amu and the atomic mass for Oxygen is 16.0 amu
- Now multiply the number of atoms found in the chemical formula with the atomic mass. 2 atoms of Hydrogen --->2(1.0) + 1atom of Oxygen-----> 16.0= 18.0 g/mol
So now that you have a basic foundation of what Molar Mass is now its
YOUR TURN to do some practice problems! Answers will be provided at the bottom of the last question. ( but don't look straight into the answers you need to TRY some problems and then check if u understand it)
:)
- What is the molar mass of NO2?
- What is the molar mass of NaCl?
- What is the molar mass of FeO?
- What is the molar mass of NaNO3?
Answers:
NO2: 14.0 + 2(16.0)= 142.1 g/mol
NaCl: 23.0 + 35.5= 58.5 g/mol
FeO: 55.8 + 16.0= 71.8 g/mol
NaNO3: 23.0 + 14.0 + 3|(16.0)= 85 g/mol
Mole Conversions
To convert between moles & mass we use molar mass as the conversion factor. You must also be sure to cancel the appropriate units.
Here's an example:
How many grams is there in 1.5 mol of 02?
1.5 Mol x
32.0 g = 48.0 g
1 mol
Now you TRY!
How many moles are there in 115g of Fe203?
A sample of HCL contains 0.54 mol. How many grams of HCl is this?