- There are SEVEN important periodic trends:
1) Reactivity
2) Ion Charge
3) Melting point
4) Atomic Radius
5) Ionization Energy
6) Electronegativity
7) Density
We will only be talking about the first 6, today!
REACTIVITY:
- metals and non-metals show different trends.
- the most reactive metal is Francium; the most reactive non-metal is fluorine.
ION CHARGE:
- Elements ion charges depend on their group (column).
MELTING POINT:
- elements in the center of the table of the highest melting point.
- noble gases have the lowest melting points.
- starting from the left to right, melting point increases, until the middle
- carbon is an exception!
ATOMIC RADIUS:
- radius decreases to the up and the right.
- helium has the smallest atomic radius.
- Francium has the largest atomic radius.
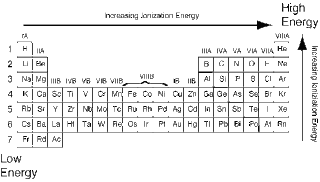
IONIZATION ENERGY:
- ionization energy is the energy needed to completely remove an electron from an atom.
- it increases going up and to the right.
- all noble gases have high ionization energy.
- helium has the highest ionization energy.
- francium has the lowest ionization energy.
- opposite trend from atomic radius.
ELECTRONEGATIVITY:
- refers to how much atoms want to gain elections.
- same trend as ionization energy.



No comments:
Post a Comment