Terms:
Acid:A solution that has an excess of H- ions. It comes from the Latin word acidus that means "sharp" or "sour".
Base: A solution that has an excess of OH- ions. Another word for base is alkali
Acids & Bases have different properties...

Classical Names:
- Ferr- Iron
- Cupp-Copper
- Mercur-Mercury
- Stann- Tin
- Aunn- Gold
- Plumb- Lead
Naming acids
*use the suffix- ic & or the prefix hydro
*Hydrogen appears first in the formula unless it is part of a polyatomic group
*use the suffix- ic & or the prefix hydro
*Hydrogen appears first in the formula unless it is part of a polyatomic group
ex: sulfuric acid
hydrochloric acid
- Hydrogen Compounds are acids
- HCl---->Hydrochloric acid
*IUPAC system was the aqeuous hydrogen compound
Acids/ Bases to memorize
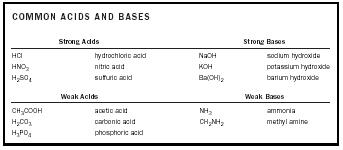
Naming Bases
*Use the cation name followed by hydroxide
- Sodium Hydroxide
- Barium Hydroxide
E.g: HI---> Iodic acid
HBR---> Hydrobromic acid
HOOCCOOH---> Oxalic acid
Here's a video that nay help you in Naming acids and Bases

- Sodium Hydroxide
- Barium Hydroxide
E.g: HI---> Iodic acid
HBR---> Hydrobromic acid
HOOCCOOH---> Oxalic acid
Here's a video that nay help you in Naming acids and Bases
Hydrates
- These crystals contain water inside them which can be released by heating.
Naming Hydrates
*These easy steps will help you ensure you get Hydrates right
1. Write the name of the chemical formula
2. ADD the prefix & # of water molecules
3. After the prefix add the hydrate
Here are some examples:

gfsgag
No comments:
Post a Comment